

The shape of ClF3 molecule is best described as. What is the formal charge of oxygen in N2O-1.
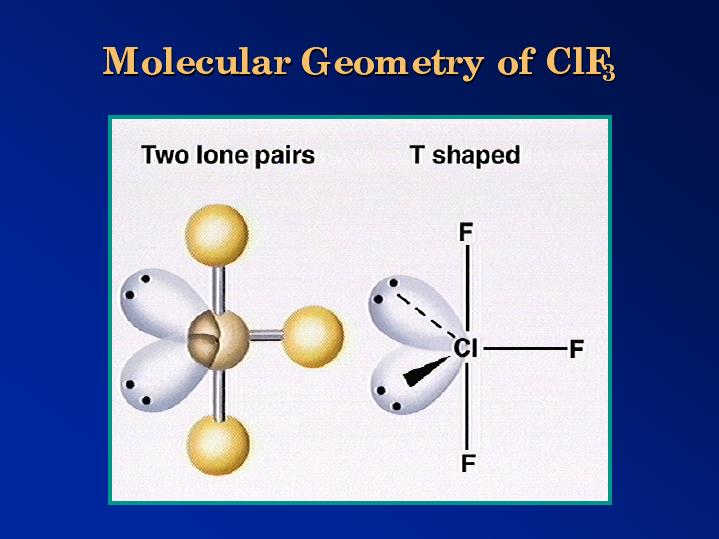
3 pts The geometry of the CIF3 molecule is best described as. What is the geometry and polarity of the CS2 molecule. A molecules molecular geometry refers to the three-dimensional arrangement of the atoms that make up the molecule. XeO3 Trigonalbipyramidal ClF3 bent T-shape XeOF4 Square pyramidal XeF2 Linear shape 13. Application of the concepts of VSEPR theory leads to the prediction that the shape of the PCl 3 molecule is A bent B linear C regular tetrahedral D triangular planar E trigonal pyramidal 9. Which of the following compounds are best described as having a T shape geometry. There is also an asymmetric charge distribution around the central atom. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom 3 bonds and 2 lone pairs. The molecule of chlorine trifluoride is as T-shaped. Contact with organic materials may result in spontaneous ignition. The study of any molecules geometry is vital since it gives data on numerous physical and chemical properties of the compounds such as polarity reactivity state of matter color magnetism organic behaviors. There are two equatorial lone pairs making the final structure T shaped. The geometry of the ClF 3 molecule is best described as. What is the geometry of PO4 3-Tetrahedral. The CS2 molecule has a linear geometry shape because it contains two sulfur atoms in the linear form and two corners with no lone pairs of electrons on central carbon atom. The shape of the ClF3 molecule is best described as A.ĬlF 3 Molecular Geometry and Shape. It reacts with water to form chlorine and hydrofluoric acid with release of heat. Cs2 Molecular Geometry Science Education And Tutorials Video Explanation Was this answer helpful.īased on the Lewis structure the number of nonbonding domains in the O 3 molecule is A 3 B 4 C 5 D 6 E 7 8.
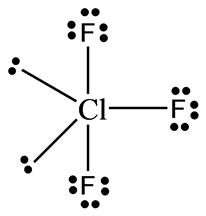
These are arranged in a trigonal bipyramidal shape with a 175 F axial-Cl-F axial bond angle. ClF3 molecular geometry is said to be T-shaped. If two bonds of trigonal biyramidal basic geometry are changed into two lone pairstherefore its molecular shape is T shape. O The molecular geometry is bent and the molecule is polar. Which molecule or ion does not have a tetrahedral molecular geometry. The geometry of ammonia molecule can be best described as. O The molecular geometry is tetrahedral and the molecule is polar. If central atoms contains 5 bond repulsion units and if it doesnt contain lone pair on central atom the shape of the molecule is trigonal bipyramidal. The shape of ClF3 molecule is T shaped The hybridisation is Sp3d. Chlorine trifluoride has 10 electrons around the central chlorine atom.Ĭlf3 Molecular Geometry Bond Angles Electron Geometry Molecular Geometry Molecular Molecules This means there are five electron pairs arranged in a trigonal bipyramidal shape with a 175 F C l F bond angle. There are two C-S double bonds at the CS2 molecular geometry.


 0 kommentar(er)
0 kommentar(er)
